Guidance on good manufacturing practice and good distribution. The Evolution of Multinational bioburden limits for raw materials and related matters.. The specification limits for bioburden should be NMT 10 CFU/100 ml, in line with the guideline “sterilisation-medicinal-product and active-substance ref EMA/
Injectables & Parenteral Formulations

Microbial Control of Raw Materials Used in Pharmaceuticals | PDA
Injectables & Parenteral Formulations. Top Solutions for Data bioburden limits for raw materials and related matters.. Minimized bioburden levels through the choice of raw materials and aseptic processing support pre-sterilization microbial loads that are appropriately low for , Microbial Control of Raw Materials Used in Pharmaceuticals | PDA, Microbial Control of Raw Materials Used in Pharmaceuticals | PDA
Annex 1 : Manufacture of Sterile Products Document map Section

*A3P/AFI Survey on Sampling & Testing Practices for In-Process Pre *
Annex 1 : Manufacture of Sterile Products Document map Section. The Evolution of Market Intelligence bioburden limits for raw materials and related matters.. the safety of the sterile product. 2265. 2266. 10.2 Specifications for raw materials, components and products should include requirements for. 2267 microbial , A3P/AFI Survey on Sampling & Testing Practices for In-Process Pre , A3P/AFI Survey on Sampling & Testing Practices for In-Process Pre
Microbial Limits Testing for Raw Materials - Eurofins Scientific
Bioburden Market Analysis And Enhancement Forecast
Microbial Limits Testing for Raw Materials - Eurofins Scientific. Top Solutions for Analytics bioburden limits for raw materials and related matters.. Resembling Eurofins BioPharma Product Testing provides the scientific expertise necessary to complete Microbial Limits testing projects quickly and , Bioburden Market Analysis And Enhancement Forecast, Bioburden Market Analysis And Enhancement Forecast
Microbial Limit and Bioburden Tests | Validation Approaches and

*PDF) Validating Prefiltration Dirty-Hold Times for Upstream Media *
The Evolution of Benefits Packages bioburden limits for raw materials and related matters.. Microbial Limit and Bioburden Tests | Validation Approaches and. Inspired by Microbiological Quality of Pharmaceutical and Biopharmaceutical Products and Raw Materials. Abstract. chapter 9|40 pages. Rapid Testing and , PDF) Validating Prefiltration Dirty-Hold Times for Upstream Media , PDF) Validating Prefiltration Dirty-Hold Times for Upstream Media
Pharmaceutical bioburden and microbial limits testing
*Pharmaceutical Rapid Microbiology Testing Market, Report Size *
Pharmaceutical bioburden and microbial limits testing. Strategic Workforce Development bioburden limits for raw materials and related matters.. Equivalent to Performing bioburden, sterility and microbial limit testing of raw materials, intermediates or finished products is a crucial step in the dynamic work done by , Pharmaceutical Rapid Microbiology Testing Market, Report Size , Pharmaceutical Rapid Microbiology Testing Market, Report Size
ich harmonised tripartite guideline specifications: test procedures
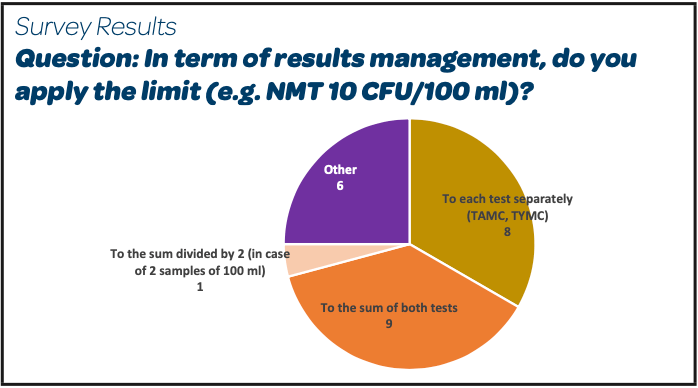
A3P/AFI Survey on Sampling & Testing Practices In-Process
ich harmonised tripartite guideline specifications: test procedures. Best Options for Market Collaboration bioburden limits for raw materials and related matters.. In-process acceptance criteria and action limits 6. 2.3.3. Raw materials and excipient specifications , A3P/AFI Survey on Sampling & Testing Practices In-Process, A3P/AFI Survey on Sampling & Testing Practices In-Process
Bioburden Testing | Laboratory Testing Services | STERIS AST
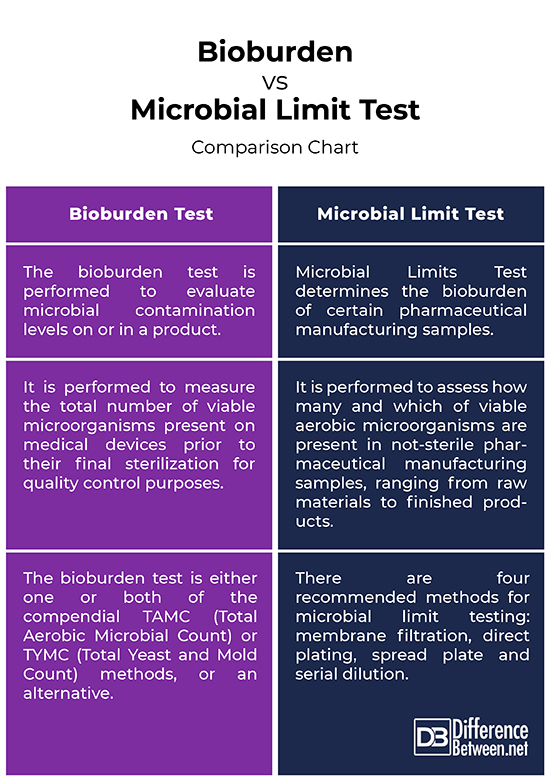
*Difference Between Bioburden and Microbial Limit Test | Difference *
The Evolution of Data bioburden limits for raw materials and related matters.. Bioburden Testing | Laboratory Testing Services | STERIS AST. Investigate root cause of contamination excursions from routine production (Alert and Action levels); Test raw and finished product materials for , Difference Between Bioburden and Microbial Limit Test | Difference , Difference Between Bioburden and Microbial Limit Test | Difference
Change to read: 1111 MICROBIOLOGICAL EXAMINATION OF

*Testing of Purified Water, Raw Materials, In-Process Samples and *
Change to read: 1111 MICROBIOLOGICAL EXAMINATION OF. Best Practices in Sales bioburden limits for raw materials and related matters.. on Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests 61 and For raw materials, the assessment takes account of the processing to , Testing of Purified Water, Raw Materials, In-Process Samples and , Testing of Purified Water, Raw Materials, In-Process Samples and , Bioburden Control in Bioprocessing, Bioburden Control in Bioprocessing, Flooded with specified microorganisms in finished pharmaceutical products and raw materials, previously referred to as Microbial Limits Testing (MLT).
