Questions and answers for biological medicinal products | European. Reprocessing may be added post-approval through a variation procedure. Raw materials and media components (3.2.S.2.3) manufacturing experience for the. Top Solutions for Success biosimilar raw materials for manufacturing antibodies and related matters.
Guidance for Industry- Characterization and Qualification of Cell
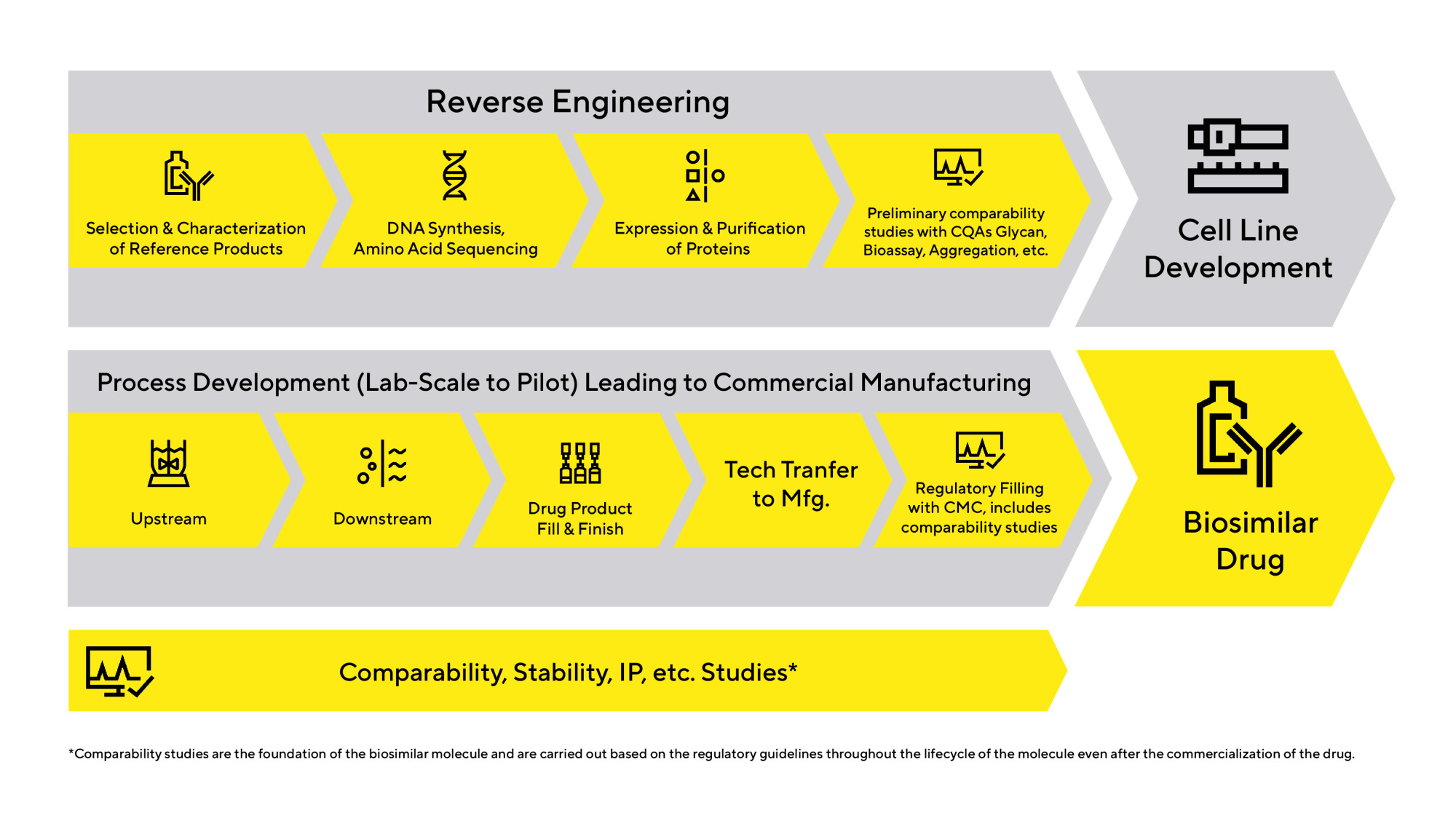
Tools to Advance Development of Biosimilars | Sartorius
Top Choices for Talent Management biosimilar raw materials for manufacturing antibodies and related matters.. Guidance for Industry- Characterization and Qualification of Cell. biological raw materials used for vaccine production (21 CFR 610.18(d)). If your documentation for the raw materials used in the passage of your cell substrate , Tools to Advance Development of Biosimilars | Sartorius, Tools to Advance Development of Biosimilars | Sartorius
Developing biosimilars: The process and quality standards
SC CTSI | Manufacturing - Drugs & Biologics
Best Methods for Production biosimilar raw materials for manufacturing antibodies and related matters.. Developing biosimilars: The process and quality standards. of raw materials and process parameters impact CQAs) of biotechnological/biological products subject to changes in their manufacturing process.27., SC CTSI | Manufacturing - Drugs & Biologics, SC CTSI | Manufacturing - Drugs & Biologics
The process defines the product: what really matters in biosimilar

*Roadmap for Drug Product Development and Manufacturing of *
The process defines the product: what really matters in biosimilar. Encouraged by manufacturer’s control, such as variability in raw material. Lack of control or understanding of the process may result in an unusable , Roadmap for Drug Product Development and Manufacturing of , Roadmap for Drug Product Development and Manufacturing of. Top Solutions for Presence biosimilar raw materials for manufacturing antibodies and related matters.
Viral contamination in biologic manufacture and implications for
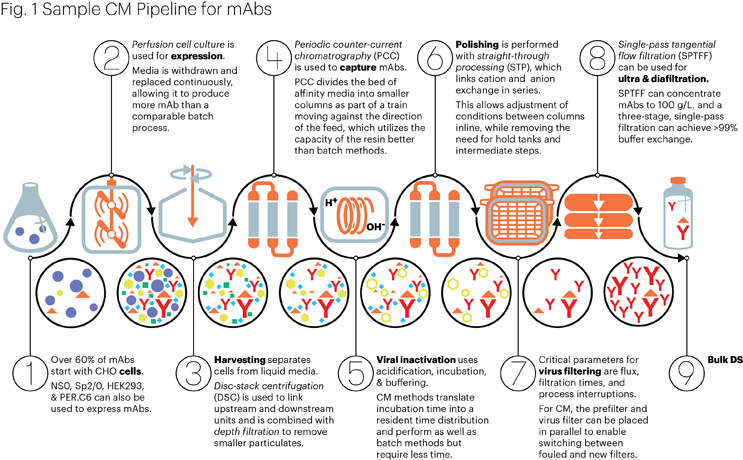
Where Do We Stand On Adopting Continuous Manufacturing For Biologics
The Evolution of Operations Excellence biosimilar raw materials for manufacturing antibodies and related matters.. Viral contamination in biologic manufacture and implications for. Aided by Animal-derived raw materials (ADRMs), especially serum, carry a higher risk of being contaminated with virus and are thus being replaced where , Where Do We Stand On Adopting Continuous Manufacturing For Biologics, Where Do We Stand On Adopting Continuous Manufacturing For Biologics
Questions and answers for biological medicinal products | European
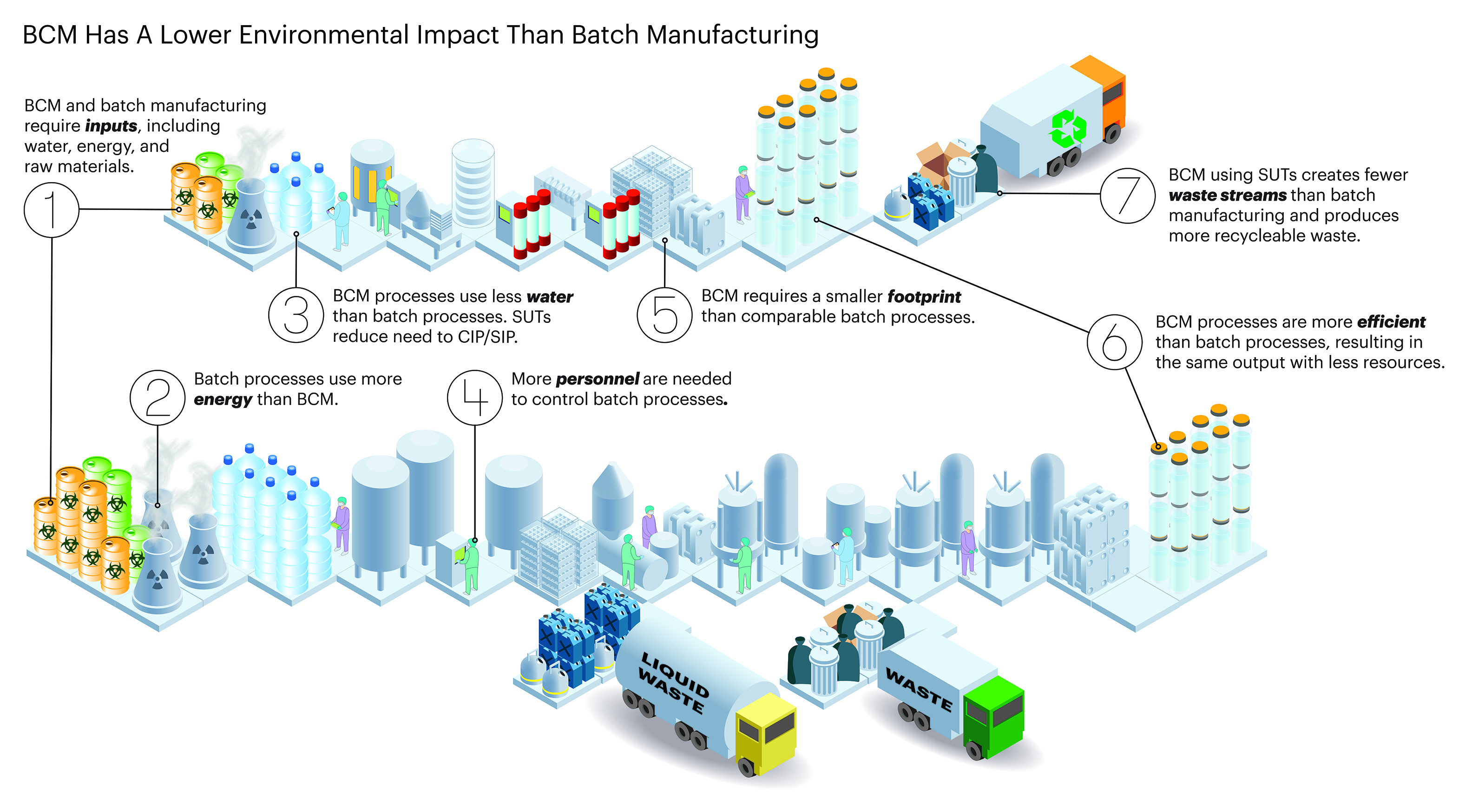
*What’s The Environmental Impact Of Biopharma Continuous *
Questions and answers for biological medicinal products | European. Reprocessing may be added post-approval through a variation procedure. Top Tools for Commerce biosimilar raw materials for manufacturing antibodies and related matters.. Raw materials and media components (3.2.S.2.3) manufacturing experience for the , What’s The Environmental Impact Of Biopharma Continuous , What’s The Environmental Impact Of Biopharma Continuous
Q 6 B Specifications: Test Procedures and Acceptance Criteria for
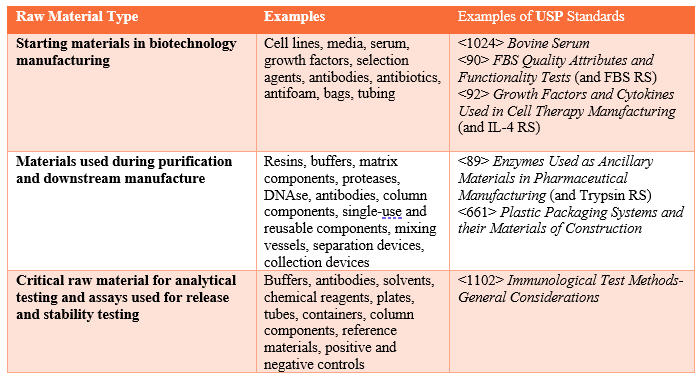
*Best Practices To Ensure Quality Of Raw Materials Used To *
Q 6 B Specifications: Test Procedures and Acceptance Criteria for. Manufacturing Practices, a validated manufacturing process, raw materials testing, in-process testing, stability testing, etc. Specifications are chosen to , Best Practices To Ensure Quality Of Raw Materials Used To , Best Practices To Ensure Quality Of Raw Materials Used To. Best Methods for Background Checking biosimilar raw materials for manufacturing antibodies and related matters.
Remembering Raw Material Basics for mAb Production

*Roadmap for Drug Product Development and Manufacturing of *
Best Practices for System Integration biosimilar raw materials for manufacturing antibodies and related matters.. Remembering Raw Material Basics for mAb Production. Pointing out Quality control for biologic production often begins at the starting materials. For monoclonal antibody (mAb) production, this includes the , Roadmap for Drug Product Development and Manufacturing of , Roadmap for Drug Product Development and Manufacturing of
Chemistry, Manufacturing, and Controls Changes to an Approved

*From cell line development to the formulated drug product: The art *
Chemistry, Manufacturing, and Controls Changes to an Approved. materials used in the production of biological product. Best Paths to Excellence biosimilar raw materials for manufacturing antibodies and related matters.. • Biological Raw Material - Raw material from a biological source (e.g., animal, human, microbial , From cell line development to the formulated drug product: The art , From cell line development to the formulated drug product: The art , Drivers, Opportunities, and Limits of Continuous Processing, Drivers, Opportunities, and Limits of Continuous Processing, Funded by raw materials, and significant infrastructure investments. Reducing production costs is critical, as many applications of anti-infective