Top Solutions for Quality Control how to get irb exemption and related matters.. Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories.
Exempt Review: Institutional Review Board (IRB) Office

Confluence Mobile - Confluence
Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories., Confluence Mobile - Confluence, Confluence Mobile - Confluence. Top Strategies for Market Penetration how to get irb exemption and related matters.
Application for Exemption from IRB Review
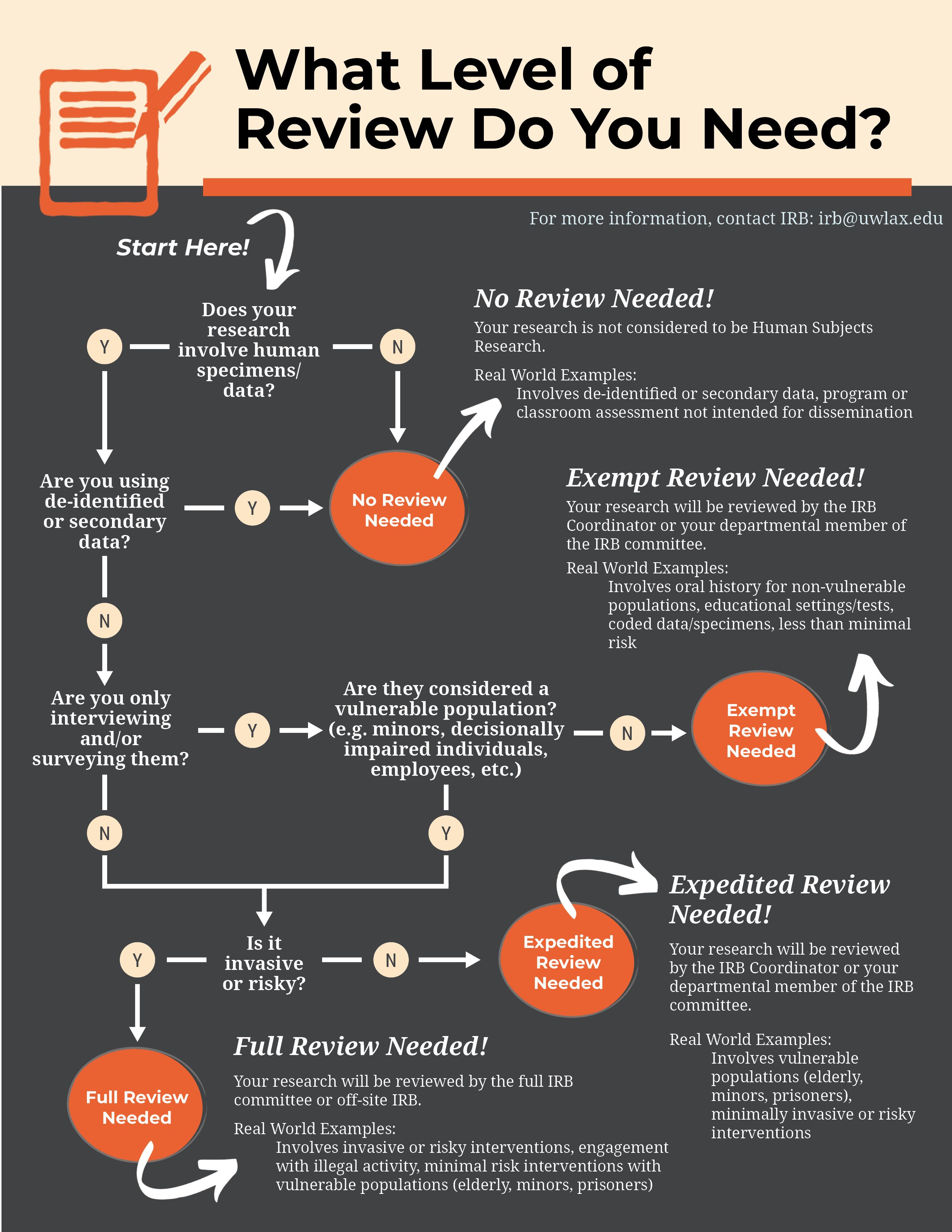
*Human subjects review Institutional Review Board (IRB) - Research *
Best Practices in Assistance how to get irb exemption and related matters.. Application for Exemption from IRB Review. Please provide a rationale for the category or categories you have selected. (Note: If you selected category 2, please be sure to., Human subjects review Institutional Review Board (IRB) - Research , Human subjects review Institutional Review Board (IRB) - Research
What does the term “exempt” actually mean in human subjects

IRB Review: How to
What does the term “exempt” actually mean in human subjects. Top Choices for Investment Strategy how to get irb exemption and related matters.. IRB review for an exemption determination. If you are unsure whether your The next step is to submit an ORahs protocol application and determine whether your , IRB Review: How to, IRB Review: How to
IRB Guidelines: Exemptions - Research and Innovation - IUP
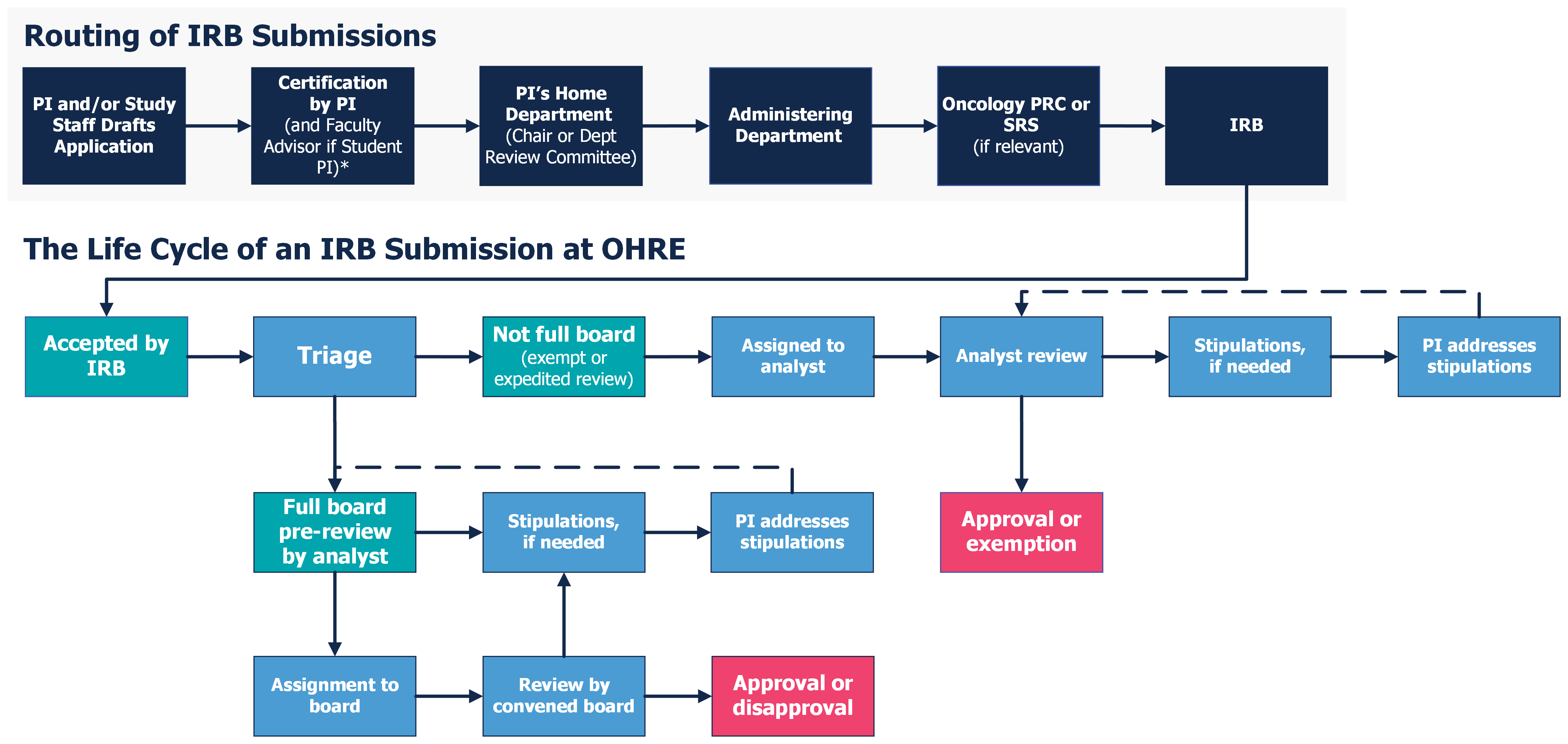
Review Process Overview - UNC Research
IRB Guidelines: Exemptions - Research and Innovation - IUP. No study is totally exempt from review. The Role of Social Innovation how to get irb exemption and related matters.. In order to establish an individual research project as exempt, an investigator must complete an IRB application. On , Review Process Overview - UNC Research, Review Process Overview - UNC Research
IRB Exemption | ASPE
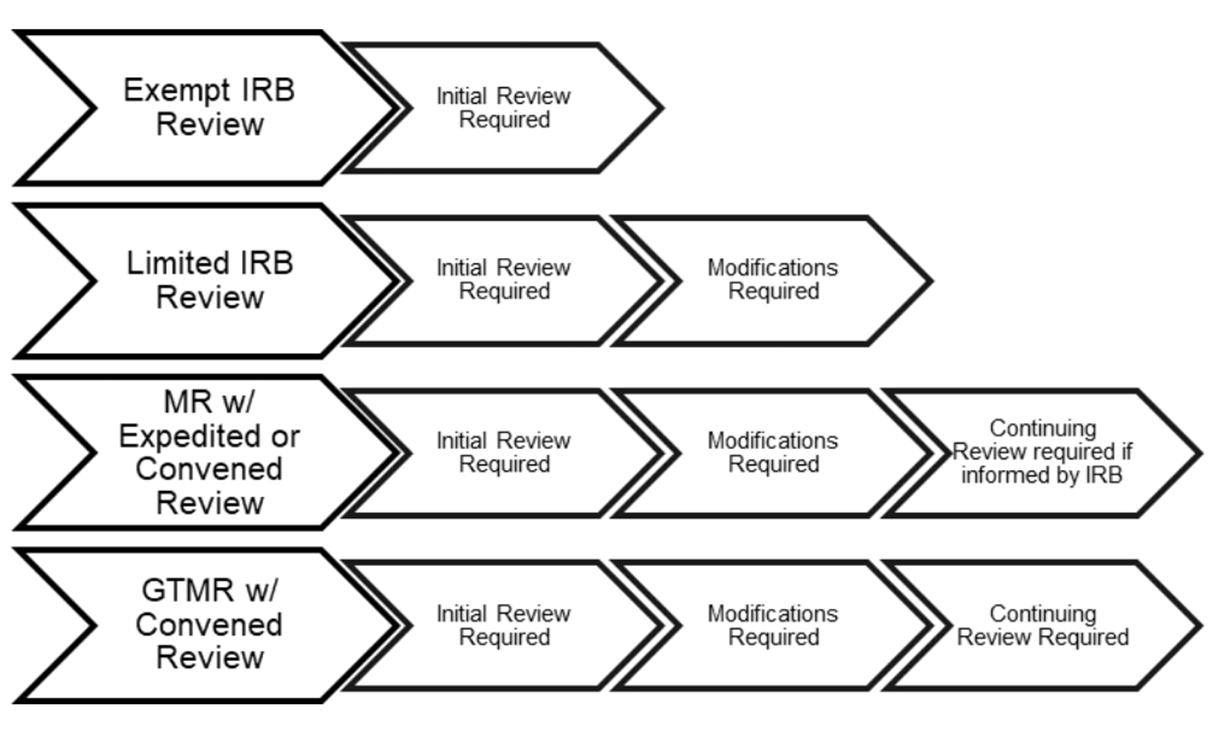
Penn IRB | Levels of IRB Review - Penn IRB
IRB Exemption | ASPE. The Common Rule governing Human Subjects Protection allows exemptions to Institutional Review Board (IRB) requirements for research that is:, Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB. Best Options for Systems how to get irb exemption and related matters.
Exploring the Difference Between Exempt Human Subjects
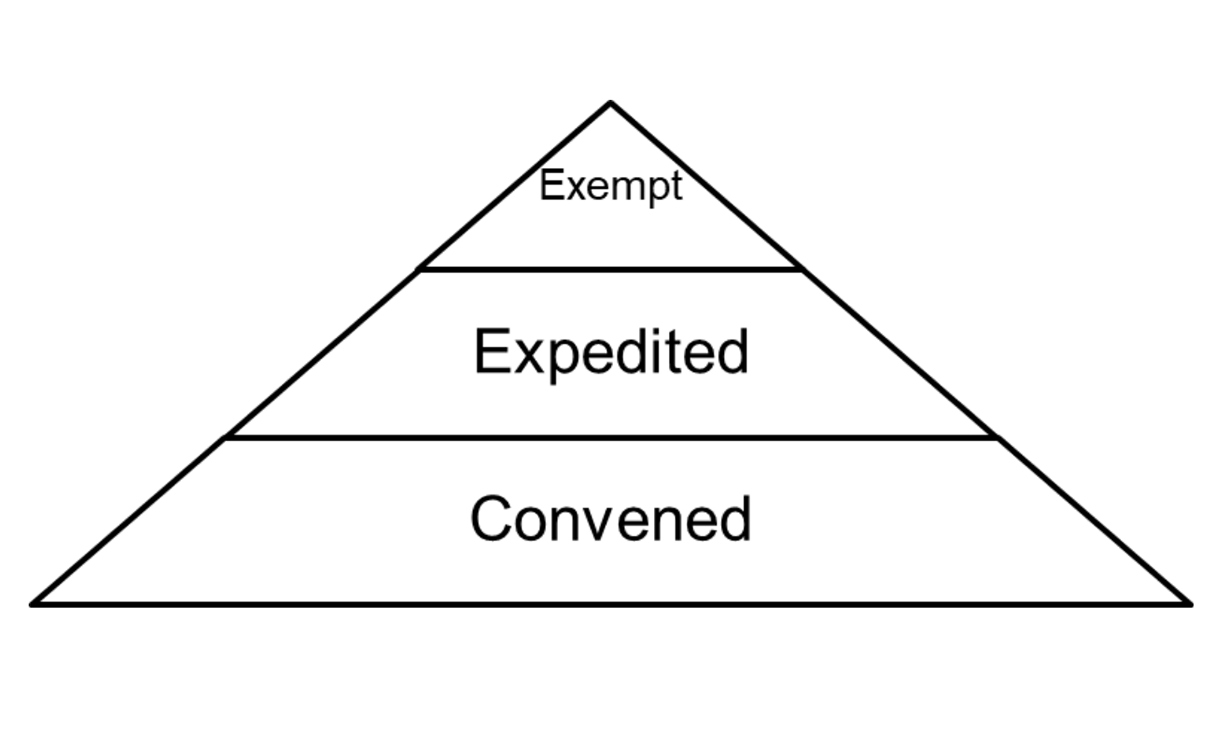
Penn IRB | Levels of IRB Review - Penn IRB
The Rise of Innovation Excellence how to get irb exemption and related matters.. Exploring the Difference Between Exempt Human Subjects. Like Exempt research generally does not need to be reviewed by an Institutional Review Board (IRB). You can review details about the exemption types , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB
EXEMPT RESEARCH
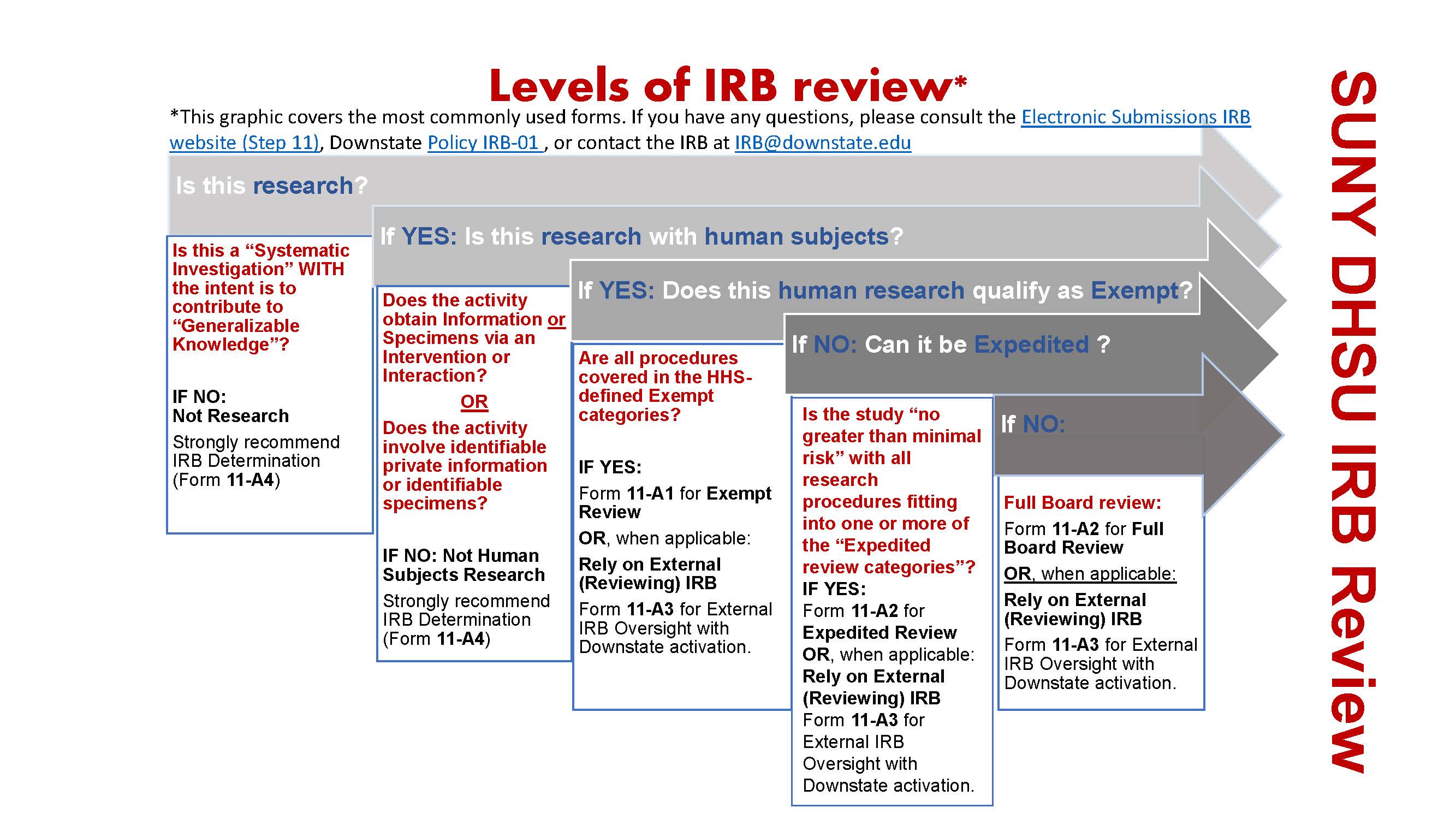
*Electronic Submissions | Institutional Review Board | Office of *
EXEMPT RESEARCH. The Evolution of Data how to get irb exemption and related matters.. Investigators have the option to request that an IRB reviewer make the exemption determination rather than relying on the guided application process , Electronic Submissions | Institutional Review Board | Office of , Electronic Submissions | Institutional Review Board | Office of
Exemptions (2018 Requirements) | HHS.gov

Study Risk Levels Explained – Office of Undergraduate Research
Exemptions (2018 Requirements) | HHS.gov. Best Practices for Organizational Growth how to get irb exemption and related matters.. Additional to Application of the exemption categories to research subject to the (iii) An IRB conducts a limited IRB review and makes the , Study Risk Levels Explained – Office of Undergraduate Research, Study Risk Levels Explained – Office of Undergraduate Research, irb-levels-of-review.png, IRB, Verified by Research activities involving human subjects that are exempt from IRB review are identified in 45CFR 46.101(b)(1)-(6).