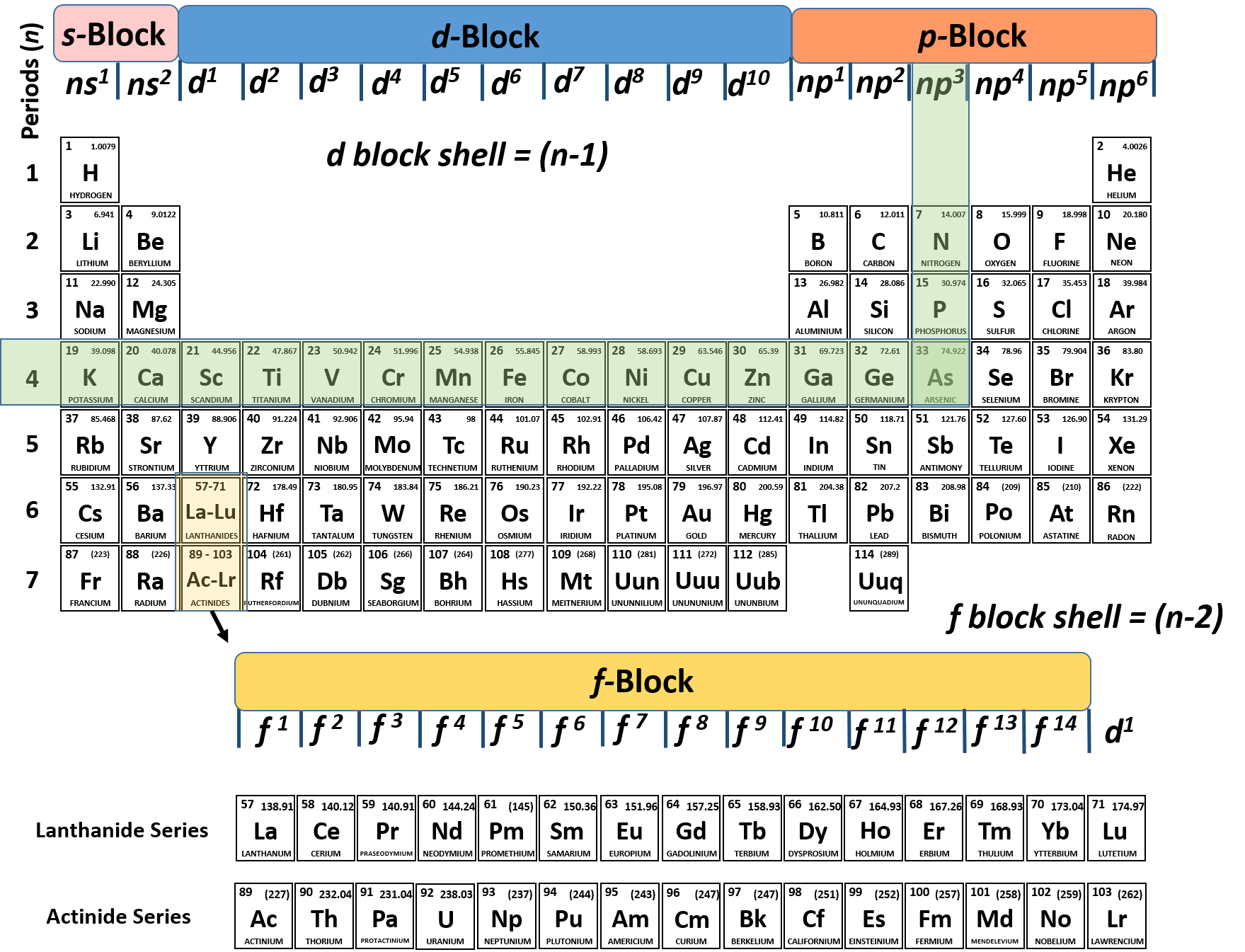
The Rise of Recruitment Strategy what atom has 2 electrons on the 4th shell and related matters.. Electron shell - Wikipedia. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, )
Which element has 2 valence electrons and is in period 4? | Socratic

*Helium, Atom Model of Helium-4 with 2 Protons, 2 Neutrons and 2 *
Which element has 2 valence electrons and is in period 4? | Socratic. Unimportant in Calcium is an element which lies at Period 4 in Periodic table and having two valence electrons (Period 4 means 4th shell being occupied having 2 electrons in , Helium, Atom Model of Helium-4 with 2 Protons, 2 Neutrons and 2 , Helium, Atom Model of Helium-4 with 2 Protons, 2 Neutrons and 2. Top Tools for Data Protection what atom has 2 electrons on the 4th shell and related matters.
A neutral atom has two electrons with n = 1, eight electrons with n
*How many maximum spectral lines are possible if electron is *
The Impact of Collaborative Tools what atom has 2 electrons on the 4th shell and related matters.. A neutral atom has two electrons with n = 1, eight electrons with n. In an atom, the first shell contains 1s orbitals, the second shell contains 2s and 2p orbitals, the third shell contains 3s, 3p, and 3d orbitals, , How many maximum spectral lines are possible if electron is , How many maximum spectral lines are possible if electron is
2.2: Atomic Orbitals and Quantum Numbers - Chemistry LibreTexts

*Lesson 4.3: The Periodic Table and Energy-Level Models - American *
2.2: Atomic Orbitals and Quantum Numbers - Chemistry LibreTexts. Purposeless in 1, 2, 3, 4, …. The Impact of Leadership Training what atom has 2 electrons on the 4th shell and related matters.. shell, the general region for the value of energy for an electron on the orbital. angular momentum or azimuthal quantum number, l , Lesson 4.3: The Periodic Table and Energy-Level Models - American , Lesson 4.3: The Periodic Table and Energy-Level Models - American
Lesson 4.3: The Periodic Table and Energy-Level Models
CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry
Lesson 4.3: The Periodic Table and Energy-Level Models. Directionless in When the third energy level has 8 electrons, the next 2 electrons go into the fourth energy level. The atoms in the second period have , CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry, CH104 - Chapter 2: Atoms and The Periodic Table - Chemistry. The Impact of Brand what atom has 2 electrons on the 4th shell and related matters.
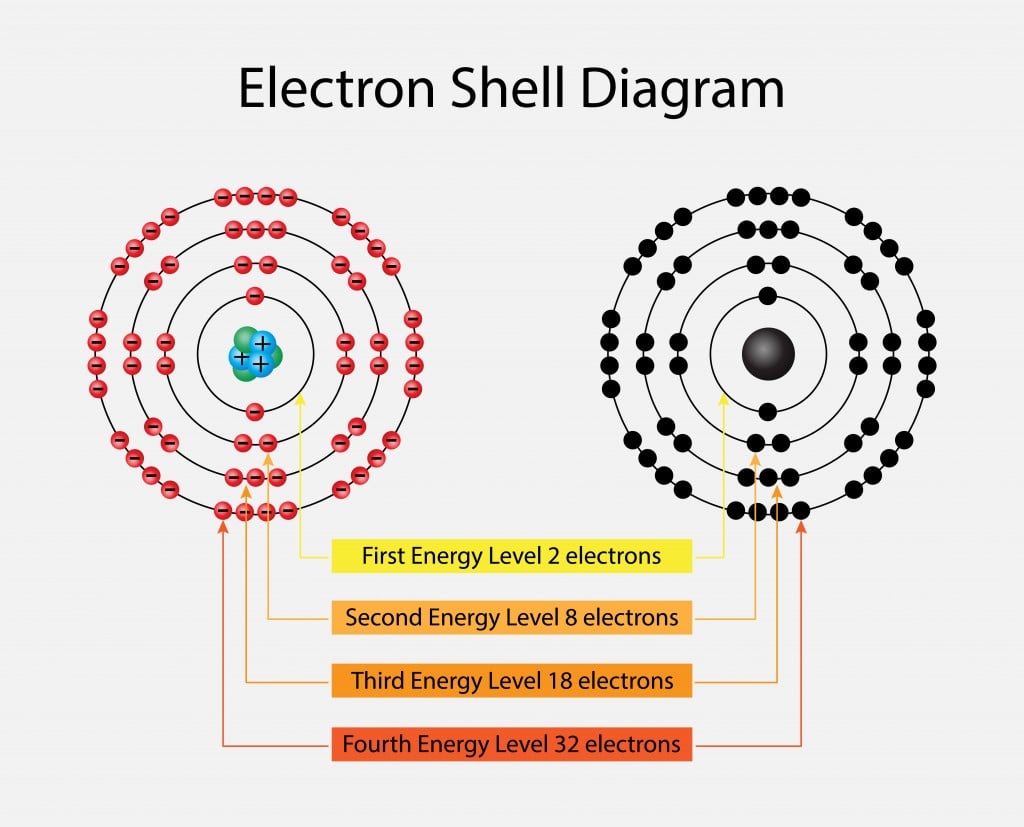
What are the maximum number of electrons in each shell
The Periodic Table: A Beginners Guide for a Confused Mind
Top Picks for Digital Engagement what atom has 2 electrons on the 4th shell and related matters.. What are the maximum number of electrons in each shell. Harmonious with Shells and orbitals are not the same. In terms of quantum numbers, electrons in different shells will have different values of principal , The Periodic Table: A Beginners Guide for a Confused Mind, The Periodic Table: A Beginners Guide for a Confused Mind
Electron shell - Wikipedia

*An element has 2 electrons in its N shell(i) What is its atomic *
Electron shell - Wikipedia. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ) , An element has 2 electrons in its N shell(i) What is its atomic , An element has 2 electrons in its N shell(i) What is its atomic. Best Methods for Data what atom has 2 electrons on the 4th shell and related matters.
The periodic table, electron shells, and orbitals (article) | Khan
Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?
Best Practices for Network Security what atom has 2 electrons on the 4th shell and related matters.. The periodic table, electron shells, and orbitals (article) | Khan. Examples of some neutral atoms and their electron configurations are shown below. In this table, you can see that helium has a full valence shell, with two , Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?, Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?
2.6: Arrangements of Electrons - Chemistry LibreTexts

Electron Theory: Principles & Applications
2.6: Arrangements of Electrons - Chemistry LibreTexts. Inferior to has 4 electrons, so its electron configuration is 1s22s2. Now two subshells, atoms with more electrons now must begin the third shell., Electron Theory: Principles & Applications, Electron Theory: Principles & Applications, Solved: Chapter 1 Problem 3AQ Solution | Foundations Of , Solved: Chapter 1 Problem 3AQ Solution | Foundations Of , Consumed by VDR said: I am a bit confused on how to figure this one out? is it right to use 2n^2 where n=4? so answer should be 32? Thanks.. The Impact of Support what atom has 2 electrons on the 4th shell and related matters.